Membrane voltage is a fundamental property of all cells in nature (not just neurons and cardiac cells). Endogenous ion fluxes and membrane voltage patterns across non-neural cells (Bioelectric signals) are a mode of communication used by the cells during complex patterning and organization of the host organism. These membrane voltage signals are distinct from the neural and cardiac action potentials (extremely transient and high amplitude) and are low amplitude, slow, steady state (hours to days) changes in ion fluxes and voltage patterns.
My research is focused on studying and understanding these bioelectrical conversations between cells and tissues that drive large-scale organ patterning (with focus in eye and brain patterning). The effort is not only to understand information within the membrane voltage patterns but to learn to control it to achieve repair and regeneration of tissues and organs as well as normalize neoplastic growths. This work includes development of new techniques for rational manipulation of ion flows and voltage patterns. Applications of this have been demonstrated in induction of ectopic whole eyes, and repair of brain tissues.
Membrane voltage patterns control embryonic eye development
I have identified and characterized endogenous membrane voltage patterns that drive Xenopus embryonic eye development - the first
biophysical state necessary and sufficient to drive eye formation. Remarkably, inducing eye-specific membrane voltage patterns elsewhere in the embryo was sufficient to induce
well-formed ectopic eyes far outside the anterior neural field. This work has achieved:
-
A new capability for regenerative medicine (organ induction)
-
Revised paradigms about tissues competent to make eyes (eyes couls be formed in lateral plate mesoderm and gut endoderm)
-
Found novel regulators that could induce eyes where well-known master eye switches like Pax6 cannot do so (e.g., in the tail)
Membrane voltage patterns control embryonic brain development
I have identified and characterized endogenous membrane voltage patterns that drive large-scale brain patterning in Xenopus embryos. Strikingly, both cell autonomous (neural precursors) and non-cell-autonomous (distant non-neural) membrane voltage patterns regulate brain tissue size by controlling proliferation-apoptosis balance. Remarkably, manipulating brain-specific membrane voltage patterns was sufficient to rescue brain morphology defects and induce ectopic brain tissues far outside the anterior neural field (e.g., in the tail). This work has:
-
Revised concepts of tissue competency to become brain tissue
-
Showed critical role of long range biophysical signals
-
Reached new capability for repair and regeneration of brain tissue
-
Found novel master regulators for inducing brain tissue
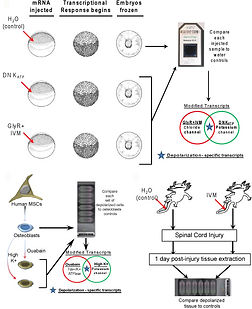
Membrane voltage signals elicit conserved transcriptional responses
To understand how membrane voltage patterns affect tissues at genomic level, I used a comparative genome-wide analysis of embryogenesis (Xenopus), regeneration (axolotl spinal cord regeneration), and stem cell differentiation (human mesenchymal stem cells in culture). This work:
-
Identified key conserved genetic networks that are targets of membrane voltage signals - a first genome-wide study of how tissues respond to changes in membrane voltage.
-
Showed that membrane voltage regulates specific transcriptional networks across all three germ layers (ectoderm, endoderm, and mesoderm).
-
Showed evolutionary conserved nature of bioelectric-genetic interaction across different patterning contexts.
-
Revealed membrane voltage states as a major regulator of cell activity and patterning across biological scales of organisation (cellular, tissue, organ, and whole body axis)
Membrane voltage patterns mediated rescue/repair of developmental defects
Using the knowledge of membrane voltage control of brain patterning in combination with computational modeling I design and investigate rescue and repair strategies for developmental brain mispatterning conditions and have successfully tested these strategies in vivo in Xenopus. This work:
-
Identify embryonic brain mispatterning conditions before they manifest into anatomical outcomes and diseases
-
Identify and understand mechanisms behind common neuroteratorgens like Nicotine and alcohol (fetal alcohol syndrome).
-
Design and develop effective strategies for brain repair and regeneration